Experiments of all shapes and sizes
In biopharma translatability of data is a serious concern, as a wide variety of sample types and experimental designs are used depending on the stage of the developmental pipeline. Pre-clinical research and discovery often use cell-based or animal-based models to test potential therapeutics, as these models allow for easier replication and large sample sizes. Due to the exploratory nature of these experiments, scientists prefer analytical methods that can measure a broad range of analytes to maximize chances of detecting a significant effect. Once researchers have identified a promising candidate drug along with relevant biomarkers for further testing in clinical trials, they might decide to switch to more targeted method to make the most of small patient biopsy samples. In addition, samples can also come in a variety of formats, whether it be formalin-fixed or flash frozen and solid tumor or serum, which will influence the effectiveness of different techniques.
Switching platforms is costly and inefficient
Unfortunately, most analytical methods are unsuitable for all scenarios, resulting in a crisis of translatability. Mass spectrometry may be a good choice for identifying biomarker candidates in cell-culture experiments but might be a poor choice in a heterogenous solid-tumor sample. To move from pre-clinical cell-culture work into animal or human biopsy experiments, one might switch to IHC to examine specific biomarkers spatially in tumor vs non-tumor tissue. However, each change in platform technology means recalibration of controls and revalidation of detection methods, costing valuable time and money.
RPPA is translatable across the development pipeline!
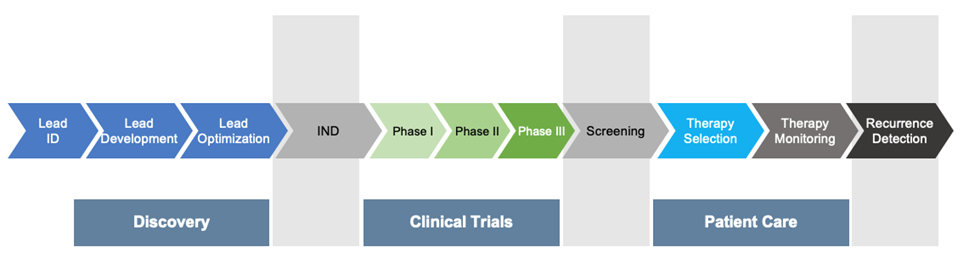
Unlike other technologies, Theralink’s RPPA platform can be used from the discovery phase through clinical trials to patient care. Whether the input material is from tissue culture, animal, or patient biopsy, flash frozen or formalin-fixed paraffin-embedded (FFPE), solid tumor or serum, the sample processing pipeline is essentially unchanged, allowing users to effortlessly transition from discovery into clinical trials and beyond without having to re-validate antibodies or switch protocols. Additionally, the high throughput of RPPA means we can analyze an entire pathway’s response, including phosphorylation states of key proteins, outperforming equivalent IHC methods. As a CLIA-certified RPPA lab, Theralink’s technology can be used as a companion diagnostic in the clinic. Our Theralink Assay for Breast Cancer has been curated for oncologists who need advanced insights into therapy options when previous treatments are no longer effective. By maintaining a consistent experience across the biopharma landscape, we ensure the translatability of data, bringing much-needed new therapeutics to market faster and more efficiently.