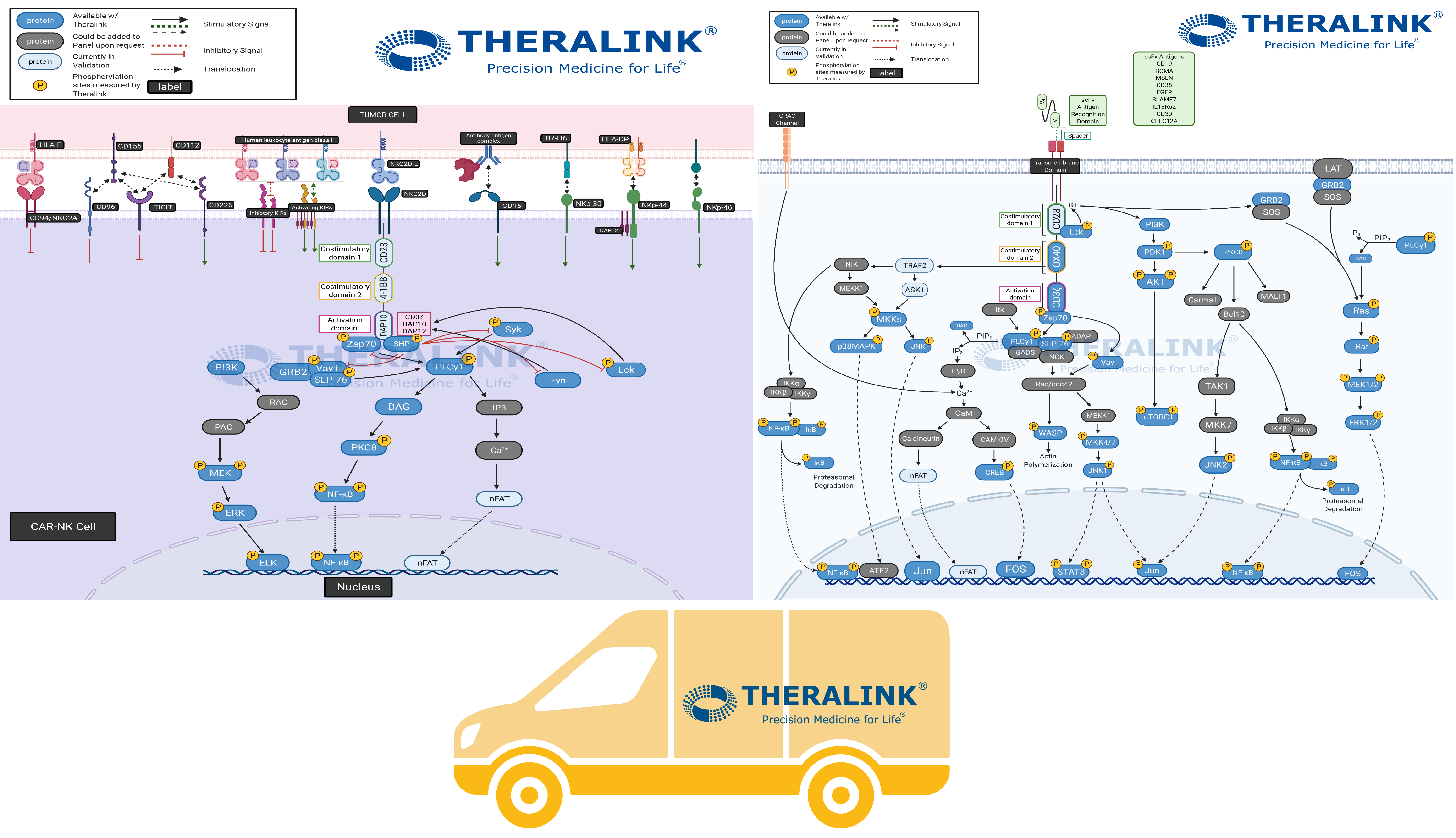
Chimeric Antigen Receptor (CAR) therapy is the modification of a patient’s immune cells so that they target cancer cells. In the case of T-cells and CAR-T therapy, cells are taken from a patient’s blood and modified to include a special receptor (CAR receptor) that binds to a specific protein on the patient’s cancer cells. Scientists grow the CAR-T cells in large quantities and put them into the patients through an infusion.
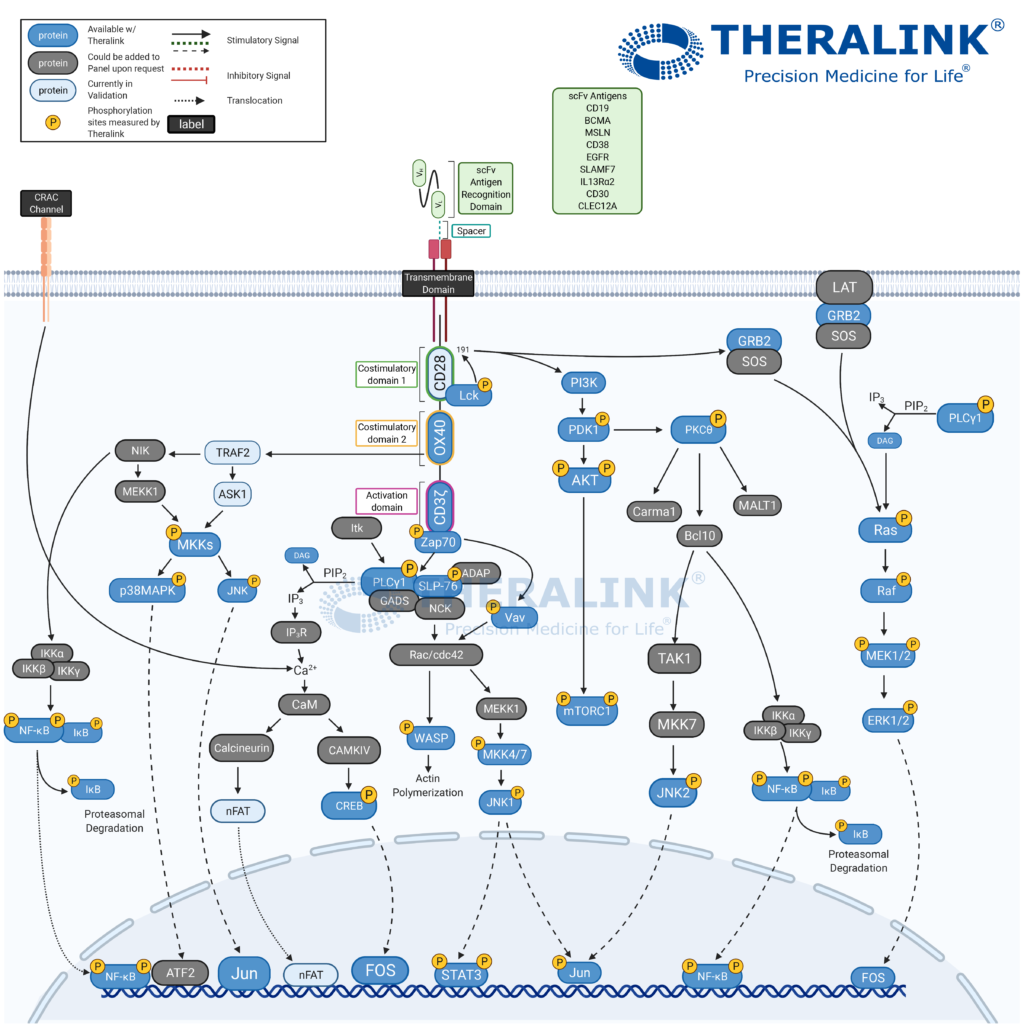
Pathway Created with Biorender.
The CAR therapy advantage is the ability to be more selective to malignant cells, consequently minimizing side effects associated with other immunotherapies. The success seen with modification to the adaptive immunity cells has led to the application of CAR therapy to the innate immunity cells- Natural Killer (NK) Cells. “T cells genetically modified with a CAR have demonstrated remarkable success in the treatment of hematological cancers. Compared to T cells, CAR-transduced NK cells (CAR-NK) exhibit several advantages, such as safety in clinical use, the mechanisms by which they recognize cancer cells, and their abundance in clinical samples.” – Hu, Yuan et al. “Chimeric antigen receptor (CAR)-transduced natural killer cells in tumor immunotherapy.”
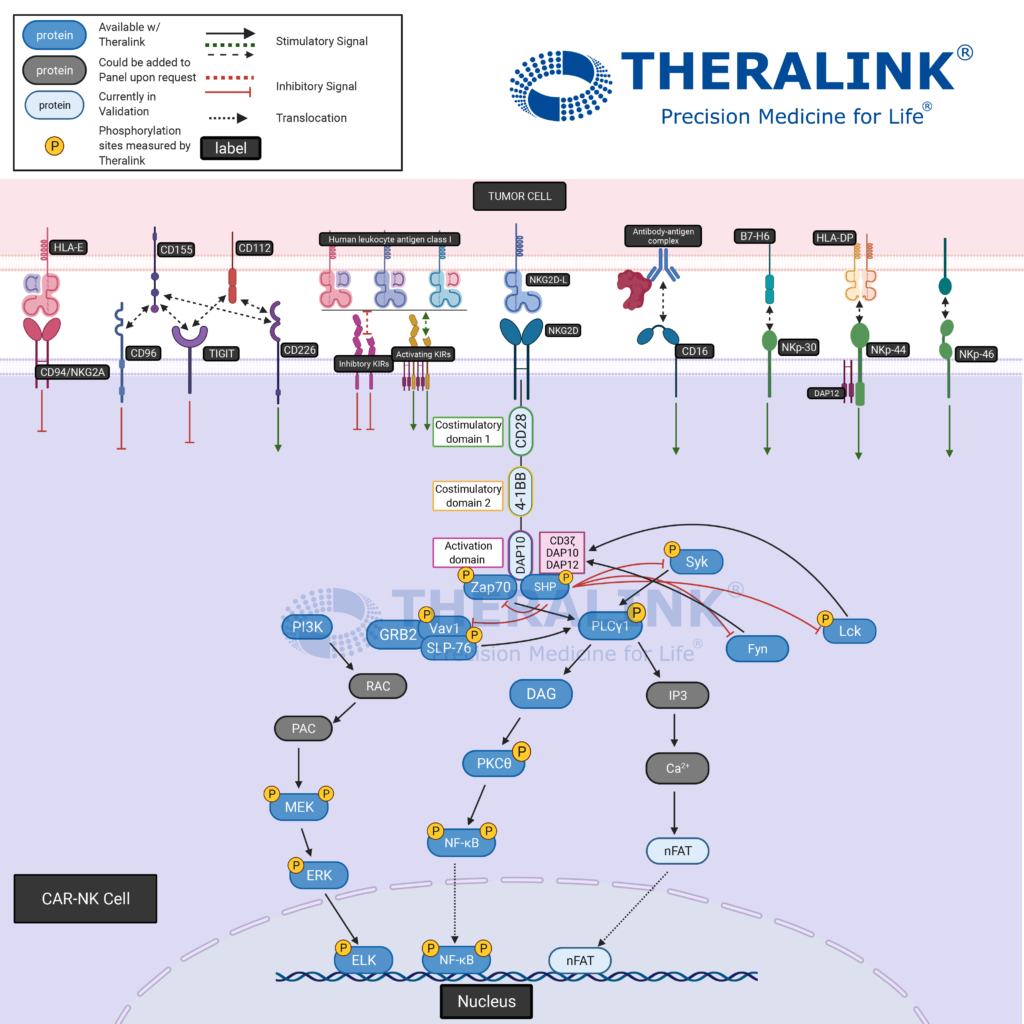
Pathway Created with Biorender
Theralink’s Next Generation Proteomics (NGP)TM platform provides customizable pathways to quantify the phosphoproteome within the tumor microenvironment and which would aid in the specification of CAR therapies. With our expanding target list (~700+ now), and several pathway panels in our arsenal, we can evaluate the therapeutic efficacy and patient suitability where it matters- protein activation. #Pathwayhighlight showcases our 700+ proteins and phosphoproteins completely customizable pathways to fit your targets of interest. Contact us for more information!
#CARNKCelltherapy #cartcelltherapy #proteomic #biomarkers #precisiononcology #oncology #science #medicine #healthcare #biotechnology #precisionmedicine #biopharma #biopharmaceuticals #healthtech #biotech #immunooncology