During experimental design talks with customers, we often hear the question, “should we fresh freeze or formalin-fix our samples?”. The decision to use fresh-frozen (FF) samples versus using a formalin-fixed paraffin embedded (FFPE) protocol is one that needs to be made early on and can result in headaches downstream if not carefully considered. Today, we’ll discuss key points to consider when deciding between the two.
FFPE allows for tissue-specific RPPA analysis
When using array-based techniques such as Reverse Phase Protein Arrays (RPPA), it’s important to know exactly what cells are being extracted for printing. While this is not a concern with cell culture tissue, samples taken from solid tumor biopsies are often a heterogenous blend of tumor and stroma. If the stroma and tumor cells are not separated via laser capture microdissection (LCM) prior to protein extraction, experimental data may be highly variable and not representative of the true tumor biology. Because of this, FFPE is the preferred method as it generally preserves the tissue morphology better than FF. With intact tissue morphology, we can perform routine histological stains and thus isolate sub-compartments (e.g., tumor from stroma) via LCM. When solid tissue is fresh-frozen, it often loses its structure, particularly after multiple freeze-thaw cycles. This makes microdissection difficult, if not impossible.
Proper antibody selection is key to high quality data
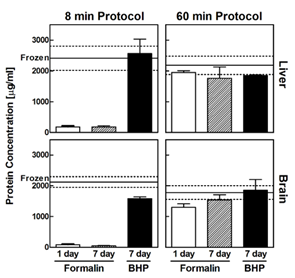
One potential advantage of FF methods is the increase in signal strength. In general, protein extraction is simpler and higher yield from FF tissue than FFPE. Mueller et al. (2011) found that protein extraction levels were much lower in FFPE compared to FF samples using a “rapid extraction protocol” (Figure 1). However, by adjusting the protocol to be longer and more stringent they were able to extract comparable protein amounts from both tissue types. Based on this, we account for the differences in FF or FFPE samples and have optimized protocols for each preservation method to ensure maximum sensitivity regardless of the input format.
More important than the overall strength is whether the change in signal after an experimental treatment is correlated between FF and FFPE samples. Several studies have shown that antibody selection has a significant effect on whether biomarker levels are correlated (Guo et al. 2012; Bader et al. 2015). Because of this, it is critical that any antibody used has been heavily tested with proper controls and the intended sample types and fixation methods. Read our article here to learn how we validate all our antibodies with experimentally.
Preserving phosphorylation status with FFPE
One of the biggest advantages of RPPA is the ability to measure phosphorylation status. As phosphorylation modifications are extremely sensitive to cell conditions, typically the gold standard for preserving phosphorylation status was flash freezing tissues immediately. However, pre-analysis variables such as time from dissection to freezing, or freeze-thaw cycles, can add variability to the results. When thinking about time from dissection to fixation using FFPE, careful consideration needs to be given to tissue size. Larger tissue sections can take hours to days to be fully fixed, during which post-translational modifications could be lost.
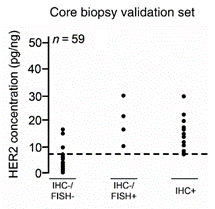
Given this, we have found that FFPE material from core needle biopsies are perfectly suitable for accurately measuring phosphoprotein levels, such as phosphoEGFR, phosphoHER2, and phosphoHER3, as used in our clinical breast cancer assay. Wulfkuhle et al. (2012) also showed that RPPA HER2 measurements from FFPE samples correlated strongly with the HER2 positivity status based on IHC and FISH methods.
Our recommendation: FFPE core needle biopsies
Based on our experience, we recommend FFPE samples from core needle biopsies or other similarly small tissue sections from clinical specimens, as this is the best balance between sensitivity, practicality, and is routine practice amongst pathology labs. While overall intensity may be higher using FF, FFPE samples are highly stable at room temperature, meaning once samples are processed, they can be easily stored for years and still accurately assayed by RPPA. FFPE also allows us to microdissect sub-compartments, again further reducing variability. Of course, if you already have FF samples, it is best to continue using the same preservation method for new samples, as the differences in signal intensity between FF and FFPE will make direct comparisons difficult.
If you are thinking of starting a new project, contact us for a consultation now! Our experienced scientists will work with you to design the optimal experimental plan, from control selection to treatment and protocol recommendations. If you already have samples and are wondering how best to proceed, we can advise you on your next best steps as well.